The neurosurgeon removes as much as is safely possible, stopping where the tumor threads into the healthy brain. Radiation and chemotherapy attack what remains. It’s exhausting, but Nadia fights through it. And when the scans come back looking promising, she even lets herself smile. Allows herself the luxury of hope. But then it returns more aggressively than before. The smile disappears. And so does any chance of hope.
This is the reality for patients facing the most aggressive malignant brain tumor, glioblastoma. Median survival rates for newly diagnosed patients sit between 12–15 months, with fewer than three in ten patients alive at the two-year mark. The standard treatment involves maximal safe surgical resection followed by concurrent radiotherapy and chemotherapy with temozolomide, which is known as the Stupp protocol.
Follow THE FUTURE on LinkedIn, Facebook, Instagram, X and Telegram
While this treatment can extend survival, it does not permanently eradicate every cancerous cell. Due to the “infiltrative growth pattern” of glioblastoma, it is impossible to surgically remove all tumor cells without causing significant neurological deficits to the patient. So, microscopic tumor cell clusters remain. Among those residual cells is a subset with stem-like properties, cancer stem cells, that resist chemo-radiation and repopulate the tumor after treatment.
Innate Repair is going after what current treatments miss. The biotech targets the Hes3 Axis, a molecular mechanism that governs when stem cells wake up to repair tissue. In cancer, it appears that the same Hes3 Axis helps tumors rebuild after therapy. By blocking this switch, the company aims to target the recurrence drivers that standard care leaves behind, to reduce relapse after treatment. The team reports Hes3 expression in more than 70 percent of glioblastoma biopsies.
In this interview with The Future Media, Dr. Andreas Androutsellis-Theotokis, CEO of Innate Repair and discoverer of the Hes3 Axis, explains how studies of brain regeneration led to an oncology strategy, why the biology may extend beyond glioblastoma, and what it takes to build a first-in-class therapeutic platform from a fundamental discovery.
How did you come into the space of cancer biology and therapeutics?
I’ve always been fascinated by regeneration — how the body repairs itself. Humans aren’t very good at it, but in nature, some animals do it remarkably well. Fish can repair serious brain injuries, and salamanders can regrow entire limbs. That inspired me to study brain regeneration in mammals, where I discovered a molecular “switch” that tells stem cells to wake up and repair damaged tissue.
At Innate Repair, we were interested in whether cancer might be using this same switch to help tumors survive and grow. If so, blocking this mechanism could give us an entirely new way to fight cancer. Our hypothesis turned out to be correct, and we are now developing therapies that shut this mechanism down to kill cancer cells. For deadly cancers like glioblastoma, where patients have very few options, this opens a much-needed new path for treatment.
What problem were you seeing in glioblastoma treatment that you felt was not being addressed?
Glioblastoma is the most common — and deadliest — primary brain tumor. Most patients live only about a year after diagnosis, even with aggressive surgery, chemotherapy, and radiation. That means we’re still missing something fundamental in how this cancer works.
Traditional treatments target the tumor’s fast-dividing cells, which can shrink it temporarily, but this is not a permanent solution. That’s because a small population of tumor cells — often called “cancer stem cells” — isn’t built to divide quickly. The job of these cells is to regenerate the tumor, and they often survive standard therapies. When they repopulate, the cancer comes back stronger and more resistant to treatment.
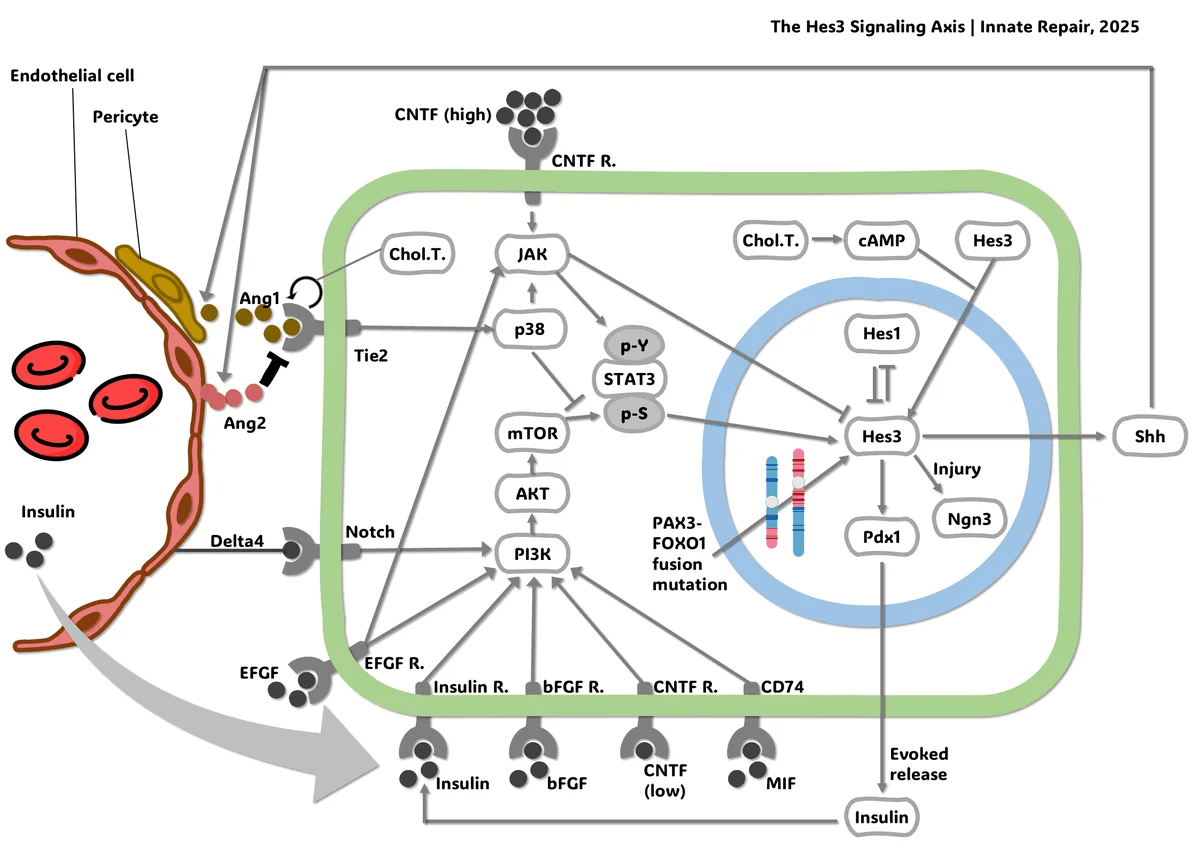
This is the gap Innate Repair is addressing. Instead of going after proliferation, which countless drugs already target, we focus on cancer’s regeneration engine — the very mechanism that allows glioblastoma to rebuild itself after therapy.
Few, if any, treatments exist here, and that’s where we believe real breakthroughs can happen.
What led you to move from research to founding Innate Repair?
My first real exposure to entrepreneurship came at the National Institutes of Health in the U.S., where I learned the importance of protecting intellectual property. Their Technology Transfer Office strongly encouraged me to patent my discoveries, and that experience shaped how I approached research later in my career. When I ran my own lab in Dresden, I made sure we secured patents around our findings as soon as the opportunity arose.
As our IP portfolio grew and the data showed genuine potential to reach patients, founding a company became the obvious next step. For me, it wasn’t enough to stay in the academic cycle of publishing and presenting. I wanted to take the leap and build something that could change lives.
Founding Innate Repair meant stepping outside the comfort of academia, taking on risk, and entering the world of entrepreneurship.
But it also meant creating a path where breakthrough science could truly make an impact, and that was a risk worth taking.
What is the main mission of Innate Repair?
At Innate Repair, our mission is to pioneer the first therapies that target the Hes3 Axis — a molecular mechanism I discovered and that plays a powerful role in both cancer and regeneration. We’re starting with glioblastoma because of its urgent, unmet need, but we and others have shown that this mechanism is also central to many other cancers.
What makes our approach unique is that the Hes3 Axis is like a biological switch. In cancer, blocking it can stop tumors from regenerating. In degenerative diseases, boosting it could help the body repair damaged tissue. So, our mission is not just to develop a new treatment for one disease, but to open an entirely new therapeutic avenue across oncology and beyond.
Ultimately, we aim to redefine how medicine thinks about both cancer and regeneration — by tackling the root mechanisms that drive disease, not just the symptoms.
What target are you pursuing, and why is it important in glioblastoma biology?
Our therapeutics fall into two categories. The first is an siRNA drug that precisely targets a key component of the Hes3 Axis. The second is a set of small molecules that are already FDA-approved for other conditions but could be repurposed for oncology. Each has its advantages: siRNA offers excellent precision but is harder to deliver, while small molecules are easy to administer but often act on multiple targets at once.
What’s exciting is that, despite these differences, both approaches lead to the same result. They selectively kill the cancer stem cells that drive glioblastoma growth — but only when the Hes3 Axis is active. That means our therapies are not indiscriminate killers; they go after the exact cells responsible for tumor regeneration, while sparing others.
This precision is crucial because glioblastoma’s resilience comes from its cancer stem cells. By targeting the root of the problem rather than just shrinking tumors temporarily, we aim for treatments with longer-lasting impact. Beyond glioblastoma, this same strategy could become a platform for tackling many other cancers that rely on the Hes3 Axis to survive.
How are you delivering the therapy?
In our pre-clinical studies, we’re focusing on intratumoral injections — delivering siRNA packaged in lipid nanoparticles directly into the tumor. It’s a precise way to make sure the therapy reaches the cancer at high enough concentrations.
Looking ahead to patients, we see two paths. One approach is to continue with intratumoral delivery, which, although surgical, is already utilised in glioblastoma care through cannulas placed directly in tumours. The second is to use new siRNA formulations being developed by others that can cross the blood–brain barrier, enabling less invasive intrathecal injections.
For our small molecules, delivery is even simpler. Some of them are already known to cross the blood–brain barrier when taken orally, which could make treatment as easy as a pill.
We aim to balance cutting-edge science with practical clinical solutions—so therapies are not only effective but also realistic to administer. And beyond glioblastoma, advances in delivery could open the door to treating many other brain diseases where drug access has always been the biggest challenge.
What evidence have you seen so far in preclinical studies?
After more than 20 years of research, we know the Hes3 Axis is a fundamental mechanism in living systems.
Boosting it enhances tissue regeneration, while blocking it reduces regeneration and increases damage. That foundation gave us the confidence to pursue it in cancer.
In oncology, our findings are especially promising:
- Over 70% of glioblastoma biopsies show Hes3 expression, confirming our target is present in patients.
- Cancer stem cells — the cells that survive current treatments — are especially dependent on Hes3.
- Our siRNA and small molecules selectively kill these cancer stem cells from multiple patients, but only when the Hes3 Axis is active.
- We’ve built stringent mouse models of glioblastoma that show high Hes3 activity, giving us clinically relevant systems to test efficacy.
- Work from our lab and others shows that the same mechanism operates in many other cancers, including breast, prostate, pancreas, lung, colon, liver, and alveolar rhabdomyosarcoma.
Together, this evidence confirms that we are hitting the right target and that the opportunity extends far beyond glioblastoma. Innate Repair is not just developing one drug for one cancer but laying the foundation for a platform approach across oncology.
Who is on your core team today?
We’re a very lean company—just three co-founders—but with an exceptional support network that allows us to operate as a virtual biotech. By co-designing and outsourcing experiments to top-tier organizations, we can channel nearly all of our resources directly into R&D.
Our founding team combines complementary expertise: myself, Dr. Andreas Androutsellis-Theotokis, as the discoverer of the Hes3 Axis; Dr. Nikos Kassapakis, a seasoned investment banker and serial entrepreneur with a PhD in nuclear physics; and Dr. Stefan Bornstein, a dean-level academic researcher who is also the director of a large university clinic. Together, we span science, business, and clinical insight.
Beyond the core team, we’ve built critical collaborations with leading institutions, including the Medicines Discovery Catapult in the UK, the University of Cambridge (Prof. George Malliaras), the Technische Universität Dresden (Medizinische Klinik und Poliklinik III), and the University of Athens Medical School (Physiology Lab, Prof. Antonios Chatzigeorgiou). These partnerships not only help us advance our therapy but also extend our science into new areas.
By combining a focused team with a world-class collaborative network, we’re able to punch far above our weight. It’s a model that allows us to advance quickly and efficiently, while laying the foundation for a platform that extends well beyond glioblastoma.
Why did you choose to participate in Startup World Cup Greece this year, and what were your main takeaways?
We joined Startup World Cup Greece because we’re actively seeking funding for the next stage of our R&D, and the event offered an excellent platform to showcase our work and gain visibility. It was also energizing to connect with other entrepreneurs and supporters of innovation — it’s always inspiring to be part of a community building bold solutions.
Our main takeaway was that sharing our story is just as important as the science itself. To bring groundbreaking therapies to patients, you need more than great research — you need investors and partners who believe in the mission.
For Innate Repair, the Startup World Cup wasn’t just about visibility, but about opening doors to those who want to be part of creating an entirely new class of cancer therapeutics.
What does success look like for Innate Repair over the next two years?
For us, success over the next two years means demonstrating clear in vivo efficacy of our therapeutics. That milestone will validate our approach and show that targeting the Hes3 Axis can truly change the course of glioblastoma — not just in cell cultures, but in living organisms.
In parallel, we aim to be in active discussions with larger pharmaceutical companies and experts in siRNA and small-molecule technologies. Building these partnerships will be key to accelerating our path to the clinic and scaling our impact.
But our vision goes beyond a single milestone. We see Innate Repair as the pioneer of a first-in-human therapeutic class — one that targets the regeneration mechanisms cancers rely on. By proving this in glioblastoma first, we open the door to a platform that can expand into many other cancers, and eventually even into regenerative medicine.
For patients, that means new hope where today there are few options. For partners and investors, it means joining us at the point where breakthrough science is ready to become transformative medicine.